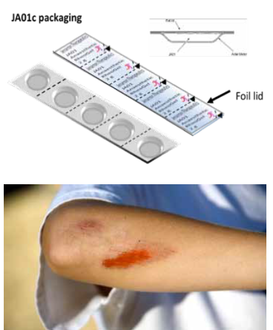
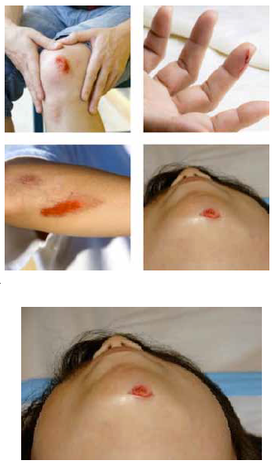
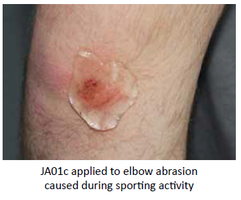
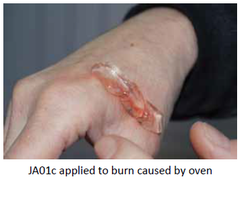
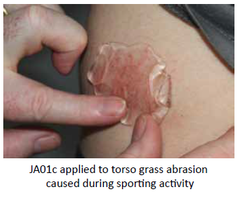
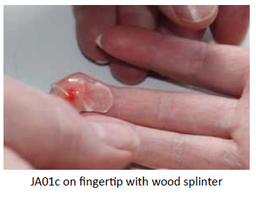
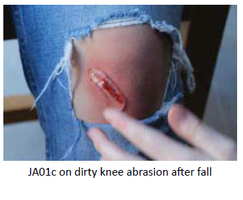
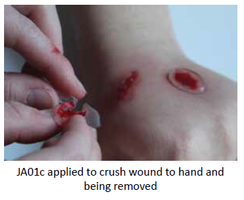
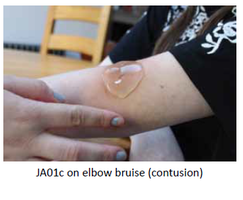
Our Product
JA01c (2g 2%) lidocaine rheogel
Potential positioning to address consumer needs and scientific support for pain relief and wound healing.
Clinical benefit and positioning.
- Pain Relief
- Protection: Immediate protection of the wound, covering it to prevent contamination.
-
Portable: Packaged ready for immediate use in the treatment of acute wounds. Minor wounds can be treated
immediately by a parent, firstaider or selfadministered in a consumer setting. - Child Friendly: No stinging on application.
-
Soothing: There is an immediate cooling and soothing effect.
-
Sealing: Prevents blood loss from small capillaries.
-
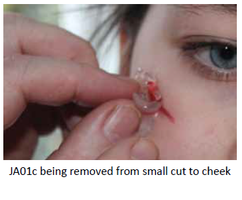
Removal: Easy to completely remove. Encapsulated contaminating debris removed.
-
Cleaning: Prevention of pain to minimise discomfort when cleaning.
-
Antiseptic: Antimicrobial action to assist in the prevention of wound infection.
-
Compatability: Compatibility with further treatment and wound healing.
How it works: Scientific Background
Lidocaine is a well-established drug used for local topical anaesthesia. There are a number of examples of lidocaine products marketed in the US with reference to an OTC monograph.
Jenarron has extensive clinical data to confirm that anaesthesia is effective and profound in open lacerations where the integrity of the skin has been
compromised.
The use of JA01c ensures a pain-free experience, especially for young children, and allows carers, such as parents, to clean and dress wounds more effectively.
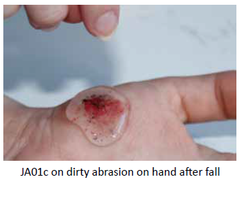
Once applied to an open wound, the rheogel flows into the cavity to form an effective barrier, preventing further wound contamination. No gaps or spaces within the wound margin exist and the tightness of fit between the wound perimeter and hydrogel is thorough.
Although the rheogel is mostly composed of water, it is dry to the touch, unlike other gel-type materials. This property gives it a structural integrity, which makes it resistant to impact and penetration.
Jenarron has extensive data showing how the rheogel flows under a wide range of applied shear conditions. These mimic flow into an acute wound, which confirms that the rheogel can self-level into abrasions and tissue trauma of various roughness and shape.
The compact uni-dose format allows it to be carried around in handbags, in the car, in first-aid kits, for outdoor sports and leisure activities, ready for immediate use.
Jenarron has packaged JA01c in a number of prototype containers and has evaluated small blister packaging, which hold samples of around 2 g.
Laboratory evaluation shows that JA01c can be easily removed from a blister made using common pharmaceutical packaging material.
Preliminary stability studies verify that the rheogel is stable in plastic and foil containers, without loss of flow or drug content.
Application of the rheogel product format is readily acceptable by children.
Application is painless. There is no stinging effect. The pH of JA01c is close to physiological. No sensation of stinging was reported when JA01 was applied to patients in the Emergency Room.
The major excipient is water which causes a cooling sensation upon application to cuts, abrasions and surrounding skin.
In human trials of rheogel prototypes conducted by Jenarron, patients report an immediate cooling sensation during application.
The rheogel forms an effective seal with underlying tissue and is able to prevent blood loss from small capillaries.
Jenarron has evaluated the adhesive strength between JA01 and a range of biological substrates. Adhesion has been shown to be considerably less than commercially available wound dressings.
Under conditions of low shear, as would exist when the rheogel is placed into a wound, JA01 possess low viscosity and completely seals any underlying surface.
Importantly, during removal, the viscosity increases substantially but there is no concurrent increase in adhesiveness.
The rheogel assists in wound cleaning. Jenarron has evaluated the ability of JA01c to remove solid debris from a range of surfaces. Data clearly show
effective removal of grit, dust, glass splinters and loose tissue. The unique flow properties enable JA01c to flow around and encapsulate foreign particles of a wide range of sizes. During removal, the rapid rise in viscosity ensures that trapped particles come away in the bulk rheogel, leaving behind a clean and particle-free surface.
Importantly, the rheogel does not infiltrate into the nascent clot, unlike fibrous materials, such as gauze. Therefore, removal of the rheogel does not disrupt the natural wound repair process.
JA01c is a non-woven hydrogel and laboratory evaluation on model wounds confirms that it does not leave remnants behind following removal. Jenarron has evaluated the adhesiveness of the rheogel and data show that it is of low adhesion and does not cause any tissue damage during removal.
As lidocaine is a rapid and effective anaesthetic agent, pain free cleaning can proceed with minimal discomfort.
Effective cleaning is essential to prevent wound infection, promote healing and minimize scar formation.
Data from clinical evaluation undertaken by Jenarron confirm that scar formation following laceration repair is minimal.
Jenarron’s patented PVA-borate rheogel formulation provides useful antimicrobial action to assist in the prevention of wound infection. It has been evaluated for anti-bacterial effects and results show that the rheogel possesses an antiseptic effect towards a selected range of common pathogens.
JA01c is a cross-linked polymeric network. Laboratory evaluations demonstrate that a small proportion of the cross-linking agent (borate anion) is able to be released from the rheogel without affecting the rheological properties. The borate anion is a pharmaceutically acceptable species that also possesses antiseptic properties.
Laboratory results show that non-drug-loaded JA01c possesses antimicrobial action. This can be enhanced by the inclusion of acceptable antimicrobial agents. Jenarron has tested a range of these payloads and has demonstrated effective and considerable antimicrobial action against bacterial colonies and biofilms.
Application of the rheogel is entirely compatible with any further necessary treatment, e.g. cleaning and wound closure, in either the consumer setting or a small injuries unit.
Extensive evaluation of JA01 in the clinical setting confirms that it is able to prepare a wound for further treatment, such as suturing or application of a plaster. As the rheogel is water-soluble, it does not leave any glue-based residues behind. JA01 does not interfere with tissue repair. JA01 has been shown in laboratory studies to absorb excess fluid, which means that it creates an ideal moist wound environment that assists in wound healing.